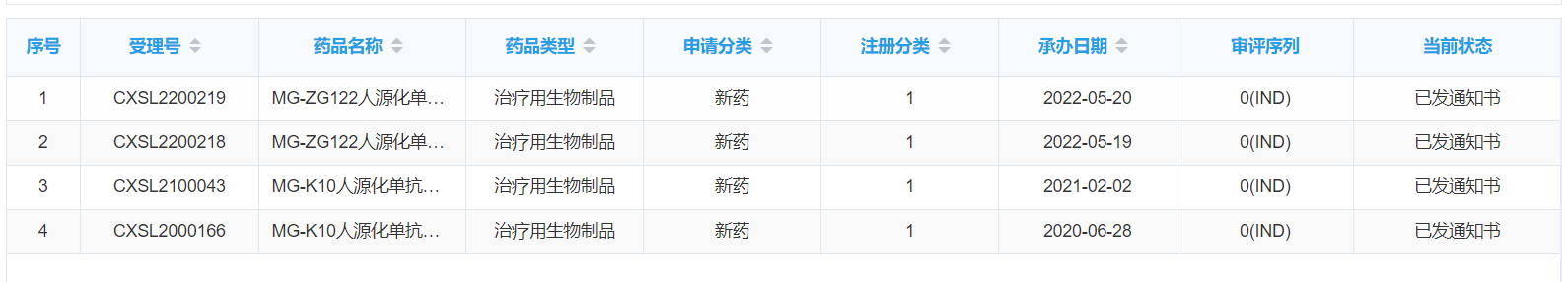
Mabgeek Biotech' second antibody drug has received the clinical trial approval notice.
2022-05-23 0
The second antibody drug "MG-ZG122 humanized monoclonal antibody injection" submitted by Mabgeek Biotech was granted the "Drug Clinical Trial Approval Notice" by the National Medical Products Administration on July 25, 2022. This product is a Class I new drug, and its indications are asthma and chronic obstructive pulmonary disease (COPD).