The second antibody drug of Mabgeek Biotech has been accepted for IND review.
2022-05-23 0
On May 20, 2022, the clinical trial application for the second antibody drug "MG-ZG122 humanized monoclonal antibody injection" submitted by Mabgeek Bio was accepted. Compared with the anti-asthma biological agents that have been marketed (such as anti-IgE antibodies, anti-IL-5 pathway antibodies, etc.), MG-ZG122 is expected to provide a more efficient and safe treatment option for non-Th2 asthma patients.
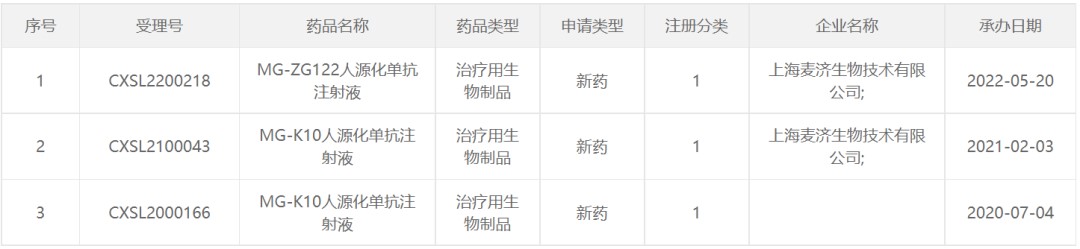
Previously, Mabgeek Bio and Anhui Wanbang Pharmaceuticals have held a discussion meeting on the Phase I clinical trial plan of MG-ZG122 humanized monoclonal antibody injection, and other new drug-related work is also being carried out. I hope that the new drug can benefit patients as soon as possible!