The phase II data of the long-acting IL-4R antibody for atopic dermatitis from Mabgeek Biotech was presented at bio Europe 2023, attracting much attention.
2023-11-09 0
The 2023 European Biotechnology Exhibition (Bio-Europe) was grandly held in Munich, Germany from November 6th to 8th. The themes of this conference include global biopharmaceutical trends and biopharmaceutical innovation directions, the rise of artificial intelligence, and the pharmaceutical transaction landscape. and successful cooperation with Big Pharma, etc.
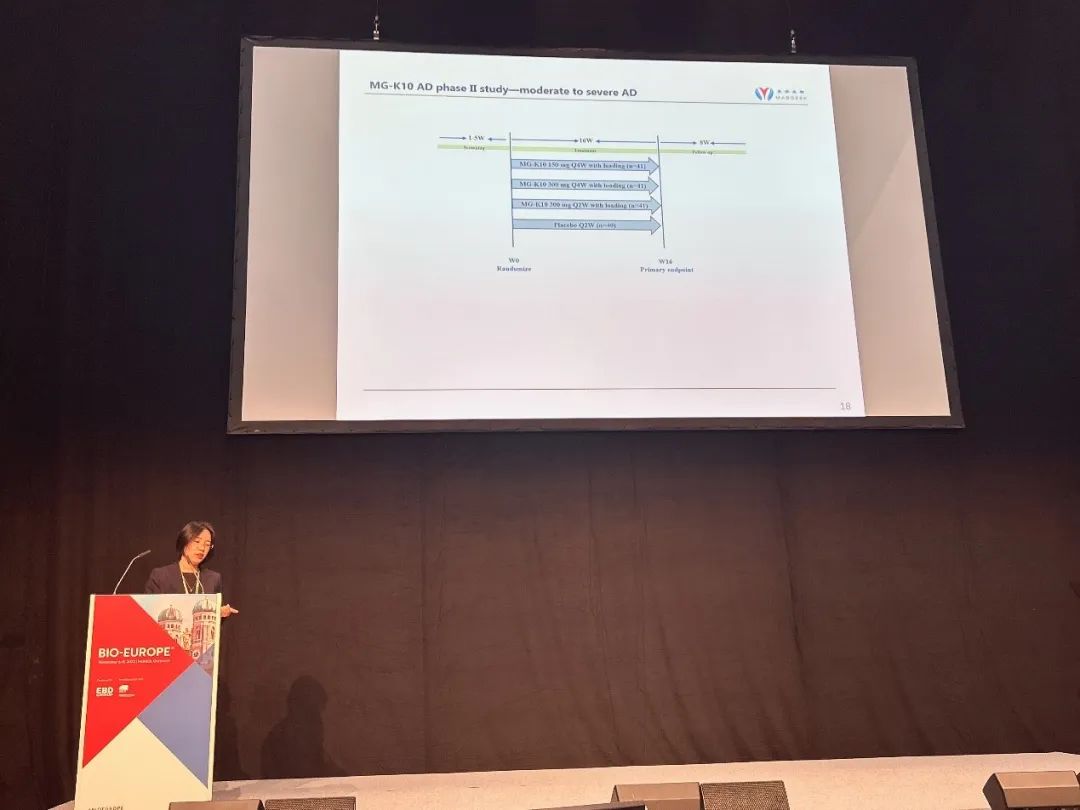

Research background


Research methods


Research results

The top-line data of the study were announced at this conference. The percentage of change from baseline in the EASI score of each dose group of MG-K10 at week 16, the primary endpoint, was higher than that in the placebo group, and the differences between the 300 mg Q4W group and the 300 mg Q2W group and the placebo group were statistically significant
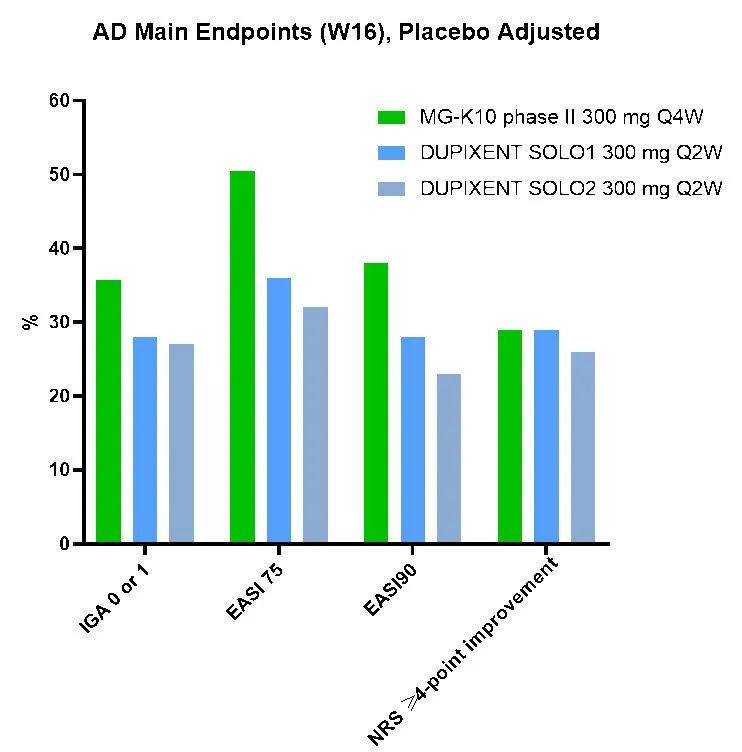
*DUPIXENT data derived from Label. Figures reflect cross-trial comparison and not results from head-to-head study. Differences exist between trial designs and subject characteristics, and caution should be exercised when comparing data across studies.

Research conclusion

MG-K10 can significantly improve the clinical symptoms of patients with moderate to severe AD and has good safety. In particular, MG-K10 has a longer Half-Life, the Phase II study showed that it can support dosing once every 4 weeks and achieve excellent therapeutic effects. It is the only long-acting anti-IL-4Rα antibody candidate drug currently on the market and in the clinical development stage that has been verified by clinical studies. At the same time, dosing once every 4 weeks can enhance patient compliance, which is more conducive to long-term control of the disease and maintaining a better quality of life.